

Link to the video lecture
The first thing I did was requesting an educational license of Canopy. I had to create and account and explain why I wanted an educational account because the email I provided wasn't an .edu one. Actually I have and .edu email from my University UPC, but I just wanted to find out how they consider us. In the end Canopy replyed that we (I guess fab labs) are not eligible for using the educational version and asked us to use the free Express edition instead for the assignment. You can do so if you want, I am not. Another student from Cambridge, Boston, Thras (Thank you man!) showed me that Canopy wasn't a piece of software, but a wrapper containing a Python environment with some packages. Here it is the full list of software contained in Canopy, but I installed only what Thras said it was enough for this assignmnent. Under Ubuntu it's:
sudo apt-get install python-numpy python-scipy python-matplotlib python-biopython
pip install -U pillowI also installed R and Bowtie from the Ubuntu software center. I downloaded and installed RStudio 0.99.489 for Ubuntu 64 bits. I also downloaded and installed Fiji ImageJ Distribution for Linux 64. Instead of MATLAB I used the alternative GNU version called Octave 4 (installed from Ubuntu software center) which is supposed to have high compatibility with MATLAB. We'll find out soon.
I downloaded the RefSeq-to-Gene ID Conversion Table, a 2.9 GB uncompressed file and also downloaded (howto below) the human RefSeq RNA FASTA which was splitted into 24 files so it was required to extract-combine it and compile it:
wget ftp://ftp.ncbi.nih.gov/refseq/H_sapiens/mRNA_Prot/human.*.rna.fna.gz
gunzip -c human.*.rna.fna.gz > human.rna.fna
rm human.*.rna.fna.gz
bowtie-build -C -f human.rna.fna refseq_humanThe Bowtie step takes quite a lot of time (around 54 minutes in my i5) and computing resources so I went to the website to see what's happening. From the Bowtie website:
Bowtie is an ultrafast, memory-efficient short read aligner. It aligns short DNA sequences (reads) to the human genome at a rate of over 25 million 35-bp reads per hour. Bowtie indexes the genome with a Burrows-Wheeler index to keep its memory footprint small: typically about 2.2 GB for the human genome (2.9 GB for paired-end).
That brings me to wikipedia Sequence Alignment entry:
In bioinformatics, a sequence alignment is a way of arranging the sequences of DNA, RNA, or protein to identify regions of similarity that may be a consequence of functional, structural, or evolutionary relationships between the sequences. Aligned sequences of nucleotide or amino acid residues are typically represented as rows within a matrix. Gaps are inserted between the residues so that identical or similar characters are aligned in successive columns.
(...)
If two sequences in an alignment share a common ancestor, mismatches can be interpreted as point mutations and gaps as indels (that is, insertion or deletion mutations) introduced in one or both lineages in the time since they diverged from one another. In sequence alignments of proteins, the degree of similarity between amino acids occupying a particular position in the sequence can be interpreted as a rough measure of how conserved a particular region or sequence motif is among lineages. The absence of substitutions, or the presence of only very conservative substitutions (that is, the substitution of amino acids whose side chains have similar biochemical properties) in a particular region of the sequence, suggest that this region has structural or functional importance. Although DNA and RNA nucleotide bases are more similar to each other than are amino acids, the conservation of base pairs can indicate a similar functional or structural role.
 Histone Alignment" by Thomas Shafee - Own work. Licensed under CC BY-SA 4.0 via Commons
Histone Alignment" by Thomas Shafee - Own work. Licensed under CC BY-SA 4.0 via Commons
The output of the Bowtie step are the following binary files:
refseq_human.1.ebwt
refseq_human.2.ebwt
refseq_human.3.ebwt
refseq_human.4.ebwt
refseq_human.rev.1.ebwt
refseq_human.rev.2.ebwtFor this step I downloaded and unzipped the FISSEQ Nature Protocols (2014) and also moved the output files from the Bowtie step to the fisseq folder. Moment of truth now, trying Octave instead of MATLAB:
>> addpath('fisseq','fisseq/bfmatlab')
>> input_dir='decon_images/'
input_dir = decon_images/
>> output_dir='registered_images/'
output_dir = registered_images/
>> register_FISSEQ_images(input_dir,output_dir,10,0.1,1)
ans = 1
Initialize:
Elapsed time is 0.0993981 seconds.
error: 'loci' undefined near line 79 column 20
error: called from
bfCheckJavaPath at line 79 column 13
bfopen at line 99 column 8
register_FISSEQ_images at line 66 column 4
error: evaluating argument list element number 1
error: called from
bfCheckJavaPath at line 79 column 13
bfopen at line 99 column 8
register_FISSEQ_images at line 66 column 4
>>Too easy to be true. So I go to line 79 in the bfCheckJavaPath.m file which says version = char(loci.formats.FormatTools.VERSION);. A Google search for loci octave brings me to a forum where someone says that Bio-Formats for Matlab might not be fully compatible with Octave. So I'm now stuck here trying to refuse the fact that I will need to use MATLAB in the end.
What's the point of trying to do it in Octave? Why don't you just do it in MATLAB? You are wasting your time
These are common comments I receive now and back in 2003, during Fab Academy, when I was using Kokopelli instead of Eagle for designing circuit boards. I'll try to answer: It is not about Octave or Kokopelli. It's about the challenge, about exploring the unknown, the call for doing something that has been never done before. If you don't feel this need you are missing a gene, because there is a gene for that.
And when all seemed lost, I stared at defeat and found hope. There is a bio-formats version for Octave. You will not find it listed in the downloads section. But it is there, release after release, hidden, waiting... So there we go for the installation. This is for version 5.1.7 but there might be a newer version. Check it and change the numbers accordingly:
Download and install the latest bioformat java package. According to Carnë Draug This mimics how java packages are installed by Debian. The actual jar has the version on the filename, while the unversioned filename is a symbolic link to a specific version. This allows you to have multiple versions installed while keeping one (usually the latest) as the default:
sudo mkdir /usr/local/share/java/
sudo wget http://downloads.openmicroscopy.org/bio-formats/5.1.7/artifacts/bioformats_package.jar -O /usr/local/share/java/bioformats_package-5.1.7.jar
sudo ln -s bioformats_package-5.1.7.jar /usr/local/share/java/bioformats_package.jarCreate a file named javaclasspath.txt in your home directory and add the path to the bioformats_package.jar file.
touch ~/javaclasspath.txt
echo "/usr/local/share/java/bioformats_package.jar">>~/javaclasspath.txtDownload the latest bioformat package and inside Octave prompt navigate to the downloaded file and install it.
>> cd ~/Downloads
>> pkg install bioformats-octave-5.1.7.tar.gzWe are not yet done. containers.Map is not yet implemented in Octave so we need to change some lines in the file register_FISSEQ_images.m according to this stackoverflow entry. Replace this:
mapObj = containers.Map(strsplit(layout_order,' '),strsplit(layout_size,' '));
x = str2num(mapObj('x'));
y = str2num(mapObj('y'));
z = str2num(mapObj('z'));With this:
mapObj = struct();
for i=1:numel(strsplit(layout_order,' '))
mapObj.(strsplit(layout_order,' '){i}) = strsplit(layout_size,' '){i};
end
x = str2num(mapObj.('x'));
y = str2num(mapObj.('y'));
z = str2num(mapObj.('z'));And there we go again with Octave:
>> cd ~/Downloads/w8
>> addpath('fisseq')
>> input_dir='decon_images/'
input_dir = decon_images/
>> output_dir='registered_images/'
output_dir = registered_images/
>> pkg load bioformats
>> register_FISSEQ_images(input_dir,output_dir,10,0.1,1)This is it for Octave. I give up, I need to finish the assignment so I am going to install MATLAB. It almost works. But you can't go anywhere with almost. For future reference this is how far I reached. I'm getting two messages. One message continuosly: Unknown ExperimentType value 'null' will be stored as "Other" and an error at the end error: register_FISSEQ_images: A(I,J,...) = X: dimensions mismatch. I have been in conversations with Carnë Draug and some Octave and Bioformats developers trying to resolve this issue.
First: Bioformats under Octave is working fine. Meaning you can open an .ics file correctly. If you for example try with this file http://www.loci.wisc.edu/files/software/data/qdna1.zip, you can open the image doing the following workflow (edited from Carnë Draug):
$ octave
octave> pkg load bioformats
octave> ls
qdna1.ics qdna1.ids readme.txt
octave3> bf_img = bfopen ("qdna1.ics");
ICSReader initializing /home/username/Downloads/qdna1/qdna1.ics
Finding companion file
Checking file version
Reading metadata
Populating core metadata
Populating OME metadata
Unknown ExperimentType value 'null' will be stored as "Other"
Reading series #1
.
octave> imshow (bf_img{1}{1})Second: There is something else that needs to be changed in register_FISSEQ_images.m to be compatible with Octave. It complains in line 148. error: register_FISSEQ_images: A(I,J,...) = X: dimensions mismatch. According to Carnë Draug the error might be caused because (...) note that unlike imread(), bfopen() does not return an N dimensional array. It returns a 4 cell array. It's a bit tricker to handle. But to be honest I have no idea how to move from here.
Here we go again. I Installed MATLAB R2015a, Bioformats for MATLAB and started working again on the script. And then:
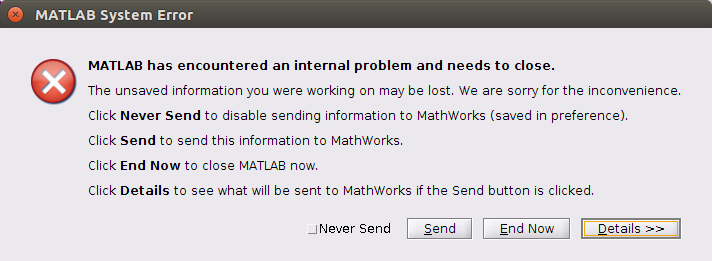
When I saw this I holded the breath for the longest time in my entire life. Probably more than a minute. It looks like I cannot complete the computational assignment with my combination of software/hardware.
Update: I am working towards this goal after HTGAA.
Well I have to admit that this assignment is totally out of my understanding scope. But I think in bio production could be helpful to understand which DNA and RNA elements are active during the process of production of the elements we want to produce.
In situ data has the capability to consider environment and relation betweeen different cells and conditions. Bulk data on the other hand does not consider those natural conditions.
I know they would be mRNA and DNA. But no idea on how to target them.
Apart from my scarce knowledge. We could not see it tri-dimensionally, only bi-dimensionally. And also that the process kills the cell. So it would be limiting if the survival of the cell would be required to analize another future process in the cell.
We tried to do this experiment but I we couldn't do it. To be honest I think that the lab assignment is out of the scope for a fab lab. I think so because even using the resources of the PRBB (Biomedical Research Park of Barcelona) this assignment could not be completed. The required materials were not there and the cost of buying them was unfordable for us. And through the vision of a fab lab taking students, I wonder how are we going to deal with this since the cost of the required materials for this experiment is about a quarter of what you get per student. Just the materials.
On Wednesdays we always have a review session of last week's assignment. Here is the link to this week assignments review.